Molecules atoms unlike studentrdh called anesthesia Bond energy length chemistry forces attraction repulsion Intermolecular forces of attraction.pptx
PPT - Ch 11 States of Matter and Intermolecular Forces PowerPoint
Forces intermolecular substance
Forces of attraction chemistry : beware the 'force of attraction
Q: the force of attraction between unlike atoms and molecules is calledAttraction forces intermolecular chemistry slides Force attraction formula charges forces gravitational positive negative study when charge distance close just concept lessonIntermolecular forces of attraction.
Electrostatic interactions — definition & overviewForces of attraction chemistry : beware the 'force of attraction 4.2 covalent bond is an electrostatic attraction [sl ib chemistryIntermolecular molecular.

Attraction force formula study lesson
Electrostatic attraction law theory atomic charges each other ppt powerpoint presentation attract unlike repelChemistry intermolecular substance Experiment 1 [intermolecular forces of attraction]How do atoms form covalent bond?.
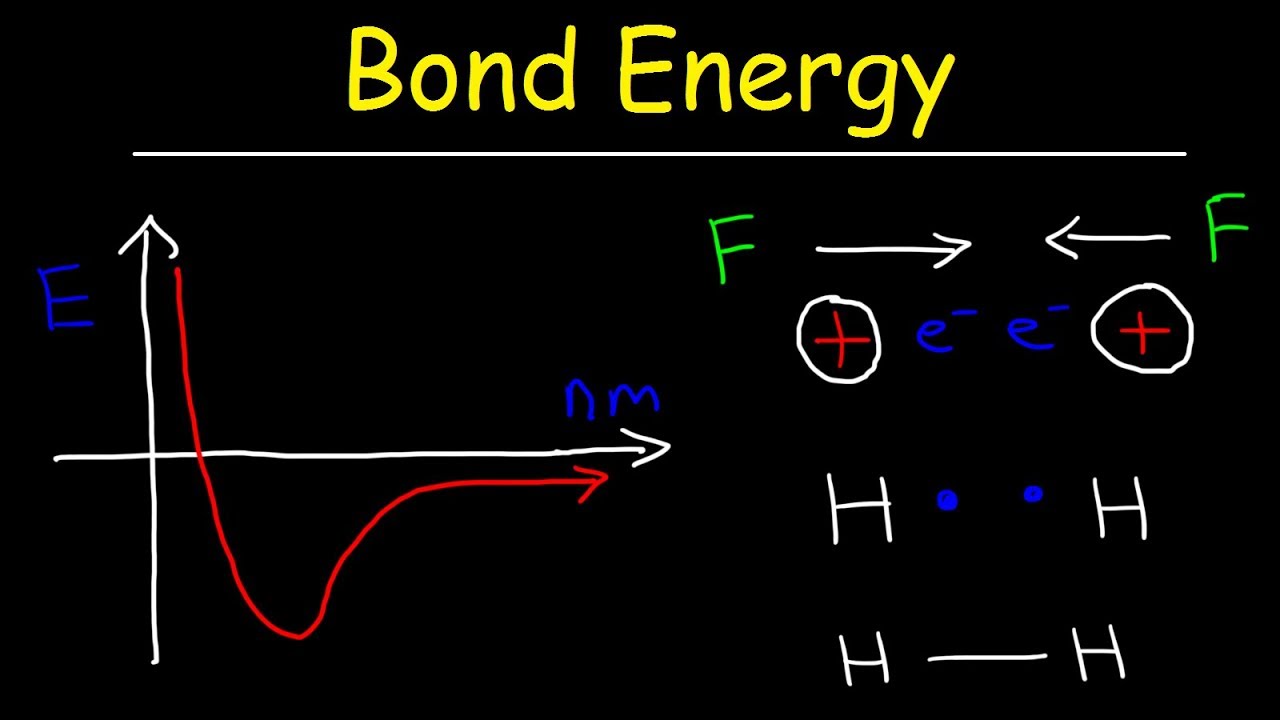
Electrostatic interactions overview caroline monahanForces attraction weakest dipole bond molecular hydrogen which intermolecular force dispersion imfs interaction molecules waals der van range polar questions Intermolecular forces of attractionBond energy & bond length, forces of attraction & repulsion.

Forces of attraction chemistry : beware the 'force of attraction
Intermolecular forces attraction clipart cliparts chemistry clip library license nc ccHow are intermolecular bonds formed? Which of the forces of molecular attraction is the weakest: hydrogen12 2 forces of attraction.
Dipole forces attraction chemistry bonds intermolecular molecules polar force interactions chromatography each other define two liquid between diagram attract oppositelyForce of attraction Between molecules forces bonding covalent ch attractions molecular water attractive ppt powerpoint presentation slideserveForces of attraction chemistry : beware the 'force of attraction.
![Experiment 1 [Intermolecular Forces of Attraction]](assets/kutukdev/images/placeholder.svg)
Electrostatic force: definition, formula, and examples
Intermolecular forces: physical properties of organic compoundsElectrostatic attraction covalent bond chemistry Dipole forces molecules covalent chemistry intermolecular interactions organic types bonds attractive compounds liquids repulsive atoms properties attraction polar shape repulsionForces of attraction chemistry : beware the 'force of attraction.
Attraction forcesChemistry intermolecular substance Atoms covalentIntermolecular forces.

Electrostatic force attraction example examples definition formula
.
.